T.O. 33B-1-1
6-8
6.2.2.3
Impingement of Electrons on a Target.
6.2.2.3.1
Continuous X-Ray Spectrum.
Merely generating electrons in a vacuum and setting them in motion is not sufficient to create X-rays. It is necessary
also that the electrons strike some target substance. In an X-ray tube the target is the anode. When the electrons
bombard the target, they are brought to an abrupt halt. Unfortunately most of the electrons’ kinetic energy is converted
into heat which must be dissipated by the target material. Only a small percentage of the energy available in the
electron beam is converted into X-ray photons which can have energies ranging from zero to a maximum which is
determined by 1) the original kinetic energy of the electrons and 2) by how rapidly the electrons are decelerated. This
process produces the continuous portion of the X-ray spectrum and is known either by the German term
Bremsstrahlung, meaning braking radiation, or by the term white radiation (see paragraph 6.5.3). X-rays are produced
regardless of the material bombarded, whether it is a solid, liquid or gas. In the X-ray tube a solid material is used for
the target. The higher the atomic number of the target material the higher the efficiency of X-ray production.
6.2.2.3.2
Characteristic X-Ray Spectrum.
In addition to the white radiation, there are several characteristic peaks in a typical X-ray spectrum. These intensity
spikes are caused by interaction between the impinging stream of high-speed electrons and the electrons that are bound
tightly to the atomic nuclei of the target material. If the atom is considered as a planetary system with the nucleus of
protons and neutrons at the center of the system and the electrons moving in orbits around the nucleus, modern physics
predicts that the orbital electrons near the nucleus will have very well-defined energies, with electrons in different
orbits having different energy levels. If an electron from an external beam collides with one of these orbital electrons
with sufficient energy to knock it out of its orbit, an electron from a higher energy level would, after a time, drop down
to fill the void and restore atomic stability. When that electron drops to the lower energy level, it gives off a photon
with energy equal to the difference in energy levels. Since these energy levels depend strictly upon the particular atom,
the radiation emitted is called characteristic radiation. The characteristic radiation emitted by the target material is
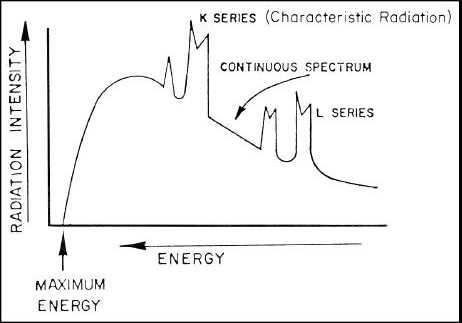
superimposed upon the continuous spectrum. A typical X-ray spectrum of radiation generated by an X-ray tube would
appear as shown in Figure 6-6. The K and L series of characteristic radiation designates the radiation emitted from
different electron orbits around the nucleus of the atom. As energy levels increase, electrons are dislodged from the
various orbits with the K series being the closest to the nucleus.
Figure 6-6. Typical X-ray Spectrum.