T.O. 33B-1-1
2-53
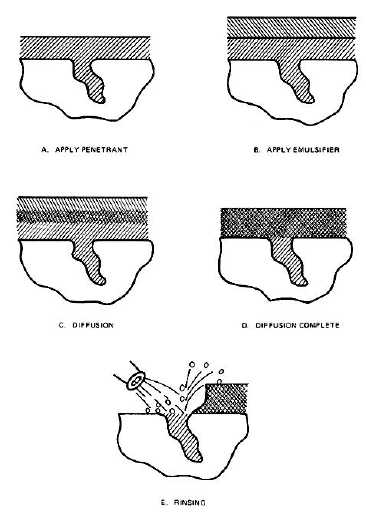
Figure 2-18. Diffusion of Emulsifier into Penetrant during the Lipophilic Emulsifier Dwell.
2.5.5.3.1.1
The physical action of the penetrant/emulsifier mixture draining from a part removes some of the excess penetrant.
The remainder of the surface penetrant is removed as the penetrant/emulsifier mixes with water during the spray rinse
and drains away. Generally oil and water do not mix, that is, they are immiscible. However, this is not always the
case. If equal amounts of oil and water are placed in a bottle, they will immediately separate into two distinct layers. If
the bottle is shaken, the oil will form into globules, which are dispersed throughout the mixture. When the bottle is
allowed to rest, the globules will rise to the surface and reform into a separate oil layer. The process of the globules
combining to form this layer is called coalescence. If the amount of oil is small compared to the quantity of water, and
the bottle is violently shaken, the oil will be broken into very small droplets. On standing, most of the droplets will
coalesce at a slower rate than previously described. However, some of the very small droplets will remain suspended in
the water giving it a cloudy or milky appearance. Depending on the droplet size, it may require an extremely long time
for separation to take place. This cloudy water mixture is called a colloidal suspension and the process by which it is
formed is termed emulsification. Certain chemicals have the ability to combine with oily materials to form an easily
emulsifiable mixture. This is the case when an emulsifier is applied to a penetrant on a part. The penetrant is oil that
repels water and resists removal. However, when combined with an emulsifier, the resulting colloidal mixture can be
removed with a water spray.